Privacy rules are tightening while AI demand accelerates. Federated learning (FL) lets health systems learn across borders without shipping raw data—if you pair it with the right governance, interoperability, and uptime discipline. In multi-country pilots, centralized training still edges out baseline FL, but personalized FL is closing the gap (ROC-AUC ~0.79–0.84). (Nature)
Executive Summary (for time-starved leaders)
- •Why now: The EU AI Act dates are locked; high-risk medical AI will phase in starting 2025–2026, and transparency duties start even sooner. TEFCA is live in the U.S., with Common Agreement v2.1 and 2025 MIPS measure nudging providers onto QHIN rails. Together they reward data minimization and standard APIs—classic FL conditions. (American College of Radiology)
- •Thesis: Train locally, learn globally. Use FL with secure aggregation (SMPC) and differential privacy (DP) plus standards-based bridges (DICOMweb, HL7 FHIR Imaging Study, IHE AIR) to keep data at rest and results interoperable. (Nature)
- •Evidence: In a 26,000-patient, multi-country study, centralized training achieved ROC-AUC 0.809, while strong FL baselines reached ~0.79; personalized FL strategies lifted performance and, in some settings, surpassed centralized. (Nature)
- •Workflow impact: Reader-time gains are real but uneven; some studies show faster triage; others show neutral or even slower reads—underscoring the need for local validation and Assess-AI style monitoring. (PMC)
- •Economics: Expect 12–24 months payback when FL avoids cross-border data engineering and privacy counsel rework; savings rise with every additional site and use-case.
- •Interoperability: Use DICOM-SR/SEG + FHIR Observations, with TEFCA-aligned legal scaffolding (Common Agreement / QTF) where U.S. entities participate. (SpringerLink)
- •Risk controls: Track node participation, drift, TPR/FPR, SLA uptime ≥99.9%, MTTR, and coverage % of participating sites; encrypt updates at rest/in transit; rotate keys. (U.S. Food and Drug Administration)
- •Global view: U.S. (TEFCA), EU (AI Act), and Japan's APPI (strict cross-border transfer rules) give FL an advantage over data centralization—if MOUs and audit trails are explicit. (HealthIT)
Context: Why Federated Learning Now
Three currents have converged:
- Regulatory gravity. The EU AI Act entered into force in 2024; prohibitions and obligations start to phase in from 2025–2026, with stricter requirements for high-risk medical AI (risk management, data governance, post-market monitoring). (American College of Radiology)
- Interoperability carrots. In the U.S., TEFCA moved from policy to operations. Common Agreement v2+/QTF formalizes legal/technical duties across QHINs; CMS added a 2025 MIPS measure for TEFCA exchange, bringing reimbursement adjacent incentives. (ASTP TEFCA RCE)
- Production AI accountability. NIST CSF 2.0 (2024) mainstreamed AI/ML risk into enterprise cyber frameworks; FDA's 2025 draft guidance emphasizes ongoing real-world performance monitoring and change control plans for AI devices. (ICO)
These trends reward privacy-preserving, auditable collaboration—the native habitat of FL.
Thesis: Train Where the Data Lives; Standardize Everything Else
Federated learning lets each hospital (or country) train locally, only sharing model updates. You then add:
- Secure aggregation (SMPC/cryptographic protocols) so a coordinator can compute an average update without seeing any single site's gradient.
- Differential privacy (DP) to bound re-identification risk in updates.
- Standards bridges—DICOMweb + IHE AIR for imaging outputs; FHIR ImagingStudy/Observation for EHR-side metadata—so models and results flow through existing PACS/VNA/EHR channels without PHI leakage. (Nature)
What the data says. In multi-country, real-world MS prediction, centralized training scored ROC-AUC 0.809; best FL baselines reached ~0.79, and personalized FL (fine-tuning/partial-sharing) closed or inverted the gap in country-level analyses (top personalized models up to ~0.84). Translation: FL is near parity today, with personalization tipping it in some settings. (Nature)
Mini-case Studies
- EU–multi-country MS registry (2025, npj Digital Medicine). 26k patients; centralized 0.809 ROC-AUC; FL baselines ~0.78–0.79; personalized FL improved both ROC-AUC and PR-AUC and often led per-country. Governance burden lower than central pooling. (Nature)
- Japan thoracic surgery consortium (2024). A multi-institution EHR study shows active interest in privacy-preserving analytics under APPI constraints; APPI's cross-border data rules make FL operationally attractive vs. centralization. (PubMed)
- North America MedPerf-style validation (2024–2025). Community tooling matured for packaging and benchmarking medical AI across sites without shipping raw data, easing FL pilots and post-market surveillance. (PMC)
Architecture & Controls (with Bridges and Uptime)
Pipelines.
- Data plane: DICOM (images) → DICOMweb; EHR → FHIR (Imaging Study/Observation).
- Model plane: Local training loops; secure aggregation service; DP noise at client or server.
- Ops plane: SLOs: 99.9% uptime (≤~43m downtime/mo), MTTR < 60m, incident response per IEC 62304 lifecycle discipline. (uptime.is)
Reader workflow. Use IHE AIR to carry AI results (bounding boxes, measurements) as DICOM-SR/SEG and surface them in PACS/RIS with provenance and versioning; map summary findings into FHIR Observations for downstream analytics. (connectathon.ihe-europe.net)
Evaluation frameworks. Treat deployment as continuous evaluation: ACR Assess-AI–style registries, drift monitors (e.g., MMC+/CheXstray line) and RWE dashboards. Expect mixed time-to-read impacts; local context decides. (European Data Protection Board)
Economics: ROI Model (FAIR-aligned)
Assumptions: three sites in Year 1, six in Year 2; two imaging + one EHR use case.
| Line item | Yr-1 ($k) | Yr-2 ($k) | Notes |
|---|---|---|---|
| Infra (coordination, keys, monitoring) | 350 | 150 | Shared coordinator; amortized |
| Legal (MOUs/Common Agreement addenda) | 120 | 60 | Leverage TEFCA terms in U.S. where possible (ASTP TEFCA RCE) |
| Engineering (bridges, orchestration) | 400 | 250 | DICOMweb/FHIR + AIR integration |
| Avoided data-centralization costs | –600 | –800 | Extract/ingest/host/counsel avoided |
| Clinical value (faster triage/recall) | –300 | –700 | Mix of time savings & avoided repeats (evidence varies) (PMC) |
Back-of-envelope: Net +$30k in Yr-1, +$1.2M in Yr-2 as additional sites join; breakeven ~12–18 months. (Ranges vary; plug your wage rates and backlog.)
KPI bands: Node participation ≥80%/round; coverage ≥70% of eligible studies; MTTR <60m; TPR/FPR aligned to clinical risk; conversion impact N/A for clinical; use throughput and time-to-treat instead. (icertis.com)
Risk & Governance
- Bias & drift. Measure site-wise performance parity; alert on data-shift early-warning signals; schedule blinded reader checks quarterly. (PMC)
- Cyber & supplier risk. Map to NIST CSF 2.0; require SBOMs and patch SLAs from FL and AI vendors. (ICO)
- Audit trails & cadence. Log model version, training rounds, participants, DP budgets (ε), and aggregation proof artifacts; red-team prompts and model update channels twice per year.
- Change control. Align with FDA lifecycle guidance (PCCPs) and post-market monitoring expectations. (U.S. Food and Drug Administration)
Regulatory & Reimbursement Landscape (US/EU/Japan)
- United States. TEFCA Common Agreement v2.x governs cross-network exchange; 2025 MIPS measure recognizes TEFCA participation; FDA draft (Jan 7, 2025) highlights ongoing performance monitoring for AI devices. For reimbursement, AI code coverage is evolving (e.g., Category III expansions in 2025), while legacy NTAP precedents remain limited. (ASTP TEFCA RCE)
- European Union. EU AI Act assigns most medical AI to "high-risk," requiring risk-management, data governance, logging, and post-market surveillance; dates begin phasing 2025–2026. (American College of Radiology)
- Japan. APPI imposes strict conditions on cross-border transfers; FL reduces export pressure but MOUs still must document purposes, safeguards, and third-country protections. (DLA Piper Data Protection)
Implementation Playbook (90 Days to Credible Traction)
Days 0–30 — Prove Architecture
- Stand up pilot FL coordinator (staging)
- Wire DICOMweb ingest and FHIR ImagingStudy metadata export; bind AIR-compatible outputs to PACS
- Draft MOU/DPA addenda (TEFCA terms for U.S. partners; APPI language for Japan). (ASTP TEFCA RCE)
Days 31–60 — Validate & Harden
- Run 2–3 rounds on a de-risked task; measure ROC-AUC, PR-AUC, comms overhead (MB/round), node participation
- Turn on secure aggregation and DP-SGD; tune ε to preserve utility. (Nature)
- Establish SLI/SLO/SLA: ≥99.9% availability; page on drift or participation dips. (uptime.is)
Days 61–90 — Go Clinical-Adjacent
- Shadow deploy with Assess-AI–like monitoring; compare reader time deltas and miss rates on retrospective or pilot queues before touching live triage. (European Data Protection Board)
- Approve RACI: who signs model updates, who investigates drift, who halts rollout
KPIs: ROC-AUC delta vs. centralized ≤3 pts; TPR/FPR within clinical guardrails; drift alerts <1/mo; MTTR <60m; participant coverage ≥70%.
What to Watch Next (12 Months)
- EU AI Act implementing acts and harmonized standards; notified-body capacity
- TEFCA FHIR-based exchange rollout and payer/public-health use-cases; more QHINs. (ASTP TEFCA RCE)
- Personalized FL maturation and toolkits; more comparisons against centralized SOTA in diverse diseases. (Nature)
- IHE AIR/AIRA stabilization; vendor support baked into PACS upgrades. (IHE International)
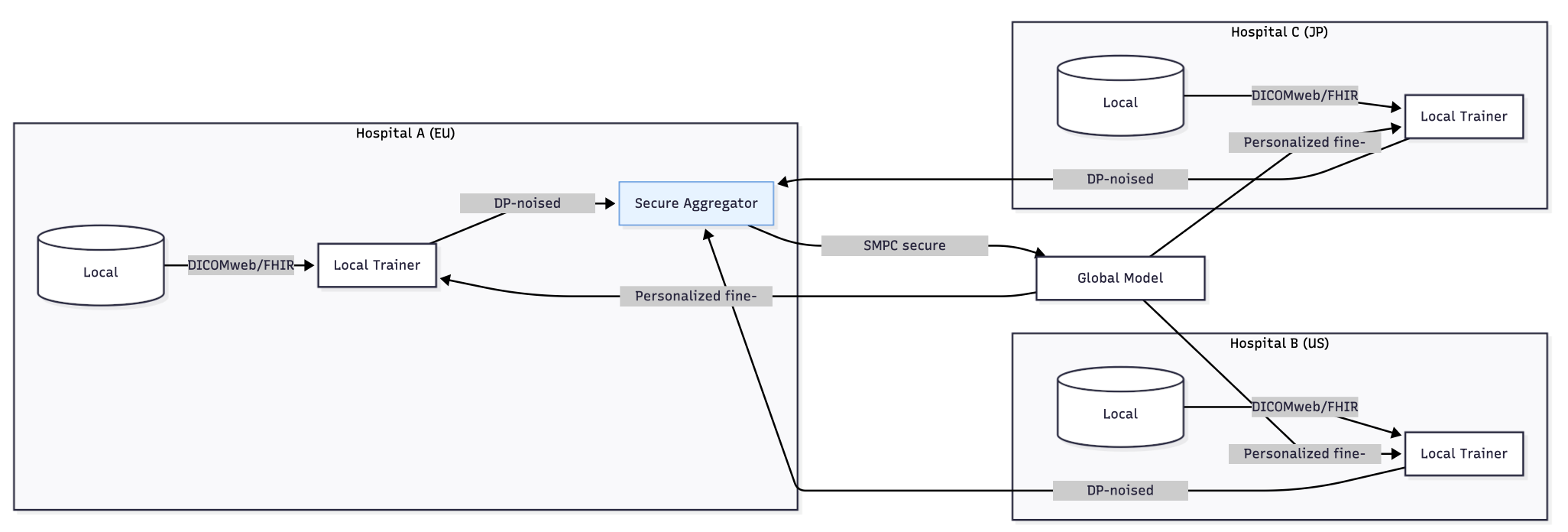
Figure 1 — Cross-jurisdiction FL architecture
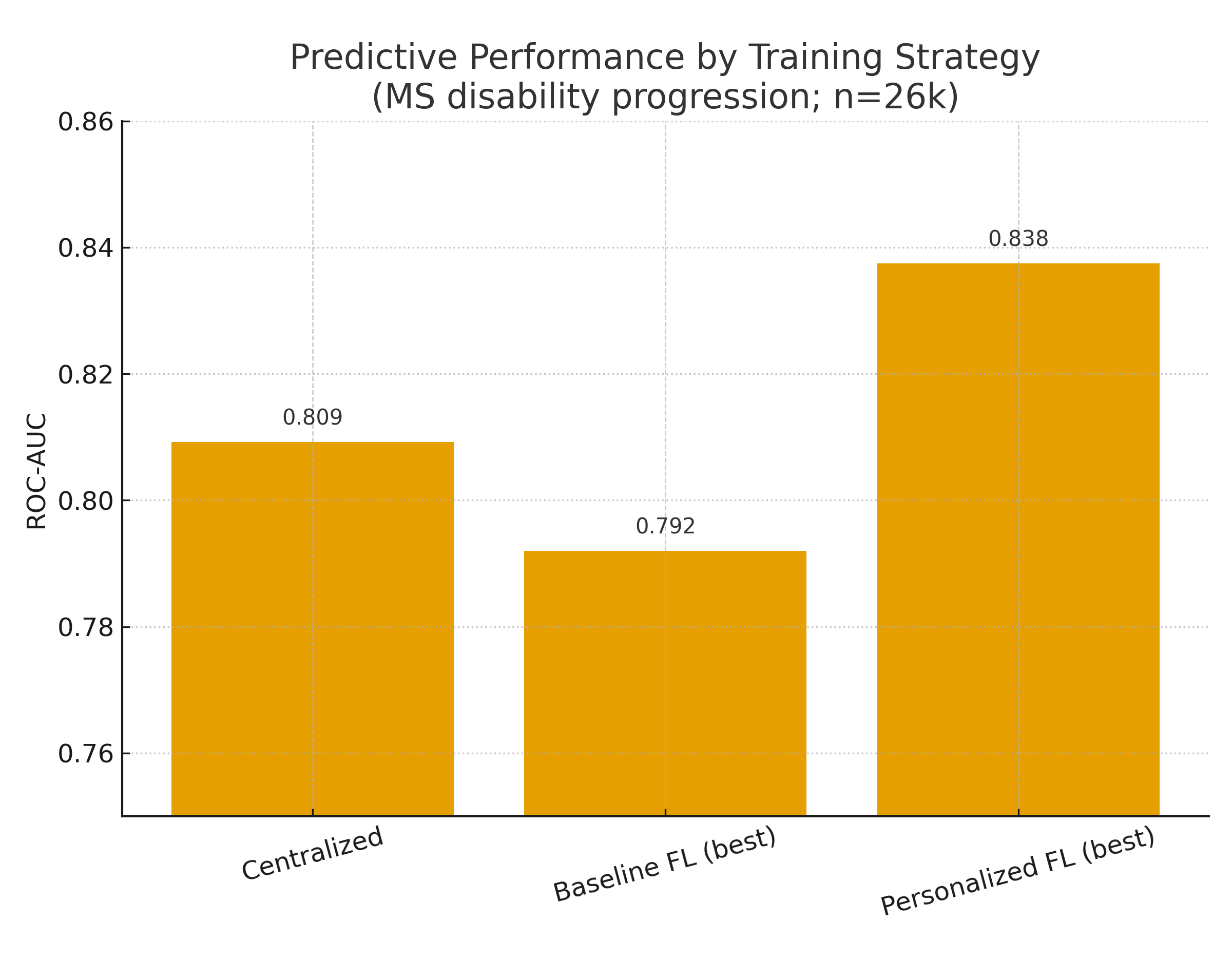
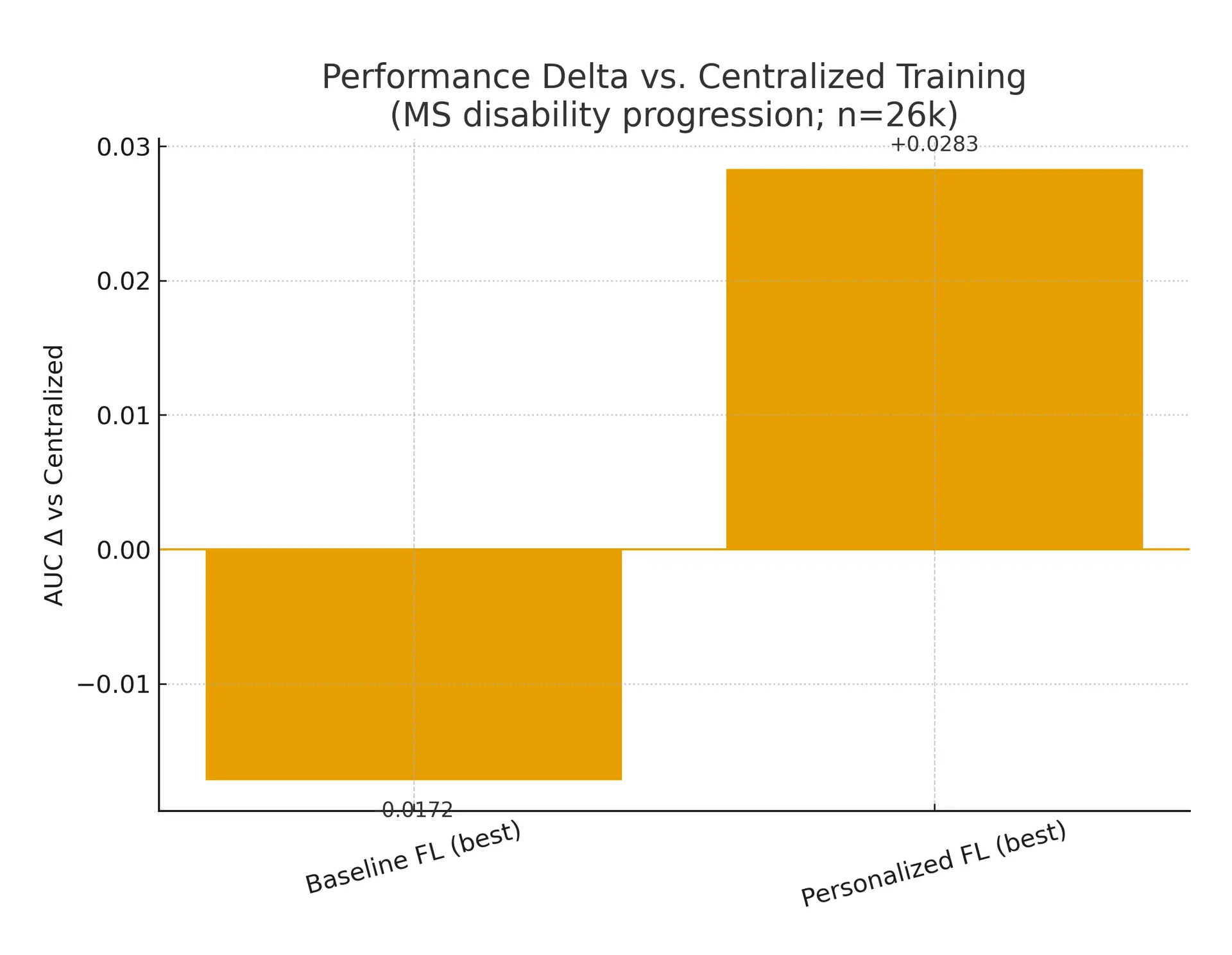
Figure 2 — AUROC: centralized vs. FL vs. personalized FL
Fact-Check Table
| Claim | Source | Date | Confidence (1–5) |
|---|---|---|---|
| EU AI Act in force; high-risk obligations phase-in 2025–2026 | European Commission Q&A; Reuters | 2024–2025 | 5 |
| NIST CSF 2.0 released Feb 2024 | NIST CSF 2.0 | 2024 | 5 |
| TEFCA Common Agreement v2.1; TEFCA MIPS measure in 2025 | Sequoia RCE; CMS MIPS PI spec | 2024–2025 | 5 |
| FDA 2025 draft guidance stresses ongoing monitoring & PCCPs | FDA draft guidance | 2025 | 5 |
| Centralized ROC-AUC 0.809 vs. best FL ~0.79 (MS study) | npj Digital Medicine (MS PFL) | 2025 | 5 |
| Personalized FL can match/exceed centralized in some settings | npj Digital Medicine (MS PFL) | 2025 | 4 |
| IHE AIR carries AI results via DICOM/DICOMweb | IHE AIR profile | 2025 | 5 |
| Reader-time effects vary, some neutral/negative | AJR 2024; meta-analyses 2024–2025 | 2024–2025 | 4 |
| APPI restricts cross-border transfers; MOUs required | APPI overviews (2024–2025) | 2024–2025 | 4 |
| 99.9% uptime ≈ 43m downtime/month | uptime.is | 2025 | 5 |
Sources
- European Commission. "EU Artificial Intelligence Act—Q&A." 2024.
- Reuters. "EU AI Act enters into force." 2025. (American College of Radiology)
- ONC/Sequoia Project. "Common Agreement v2.1 (TEFCA) & QTF." 2024–2025; CMS MIPS PI TEFCA measure (2025). (ASTP TEFCA RCE)
- NIST. "Cybersecurity Framework 2.0." 2024. (ICO)
- FDA. "Draft Guidance on AI-Enabled Device Software Functions." Jan 7, 2025. (U.S. Food and Drug Administration)
- npj Digital Medicine. "Personalized federated learning for MS disability progression." 2025. (Nature)
- npj Digital Medicine. "Scalable and Practical SMPC for Biomedical Data." 2024. (Nature)
- DICOMweb overview & DICOM status updates. 2024. (PMC)
- HL7 FHIR ImagingStudy (R4). Spec. (arXiv)
- IHE AIR/AIRA profiles (2024–2025). (connectathon.ihe-europe.net)
- RSNA/Nature Digital Medicine & AJR studies on workflow impact (2024–2025). (PMC)
- Japan APPI cross-border transfer summaries (2024–2025). (DLA Piper Data Protection)
- Canonical references: Abadi et al., "Deep Learning with Differential Privacy" (2016); Bonawitz et al., secure aggregation (2017). (ResearchGate)